
Next-Generation Literature Management
Boost Your Literature Management with AI-Driven Support
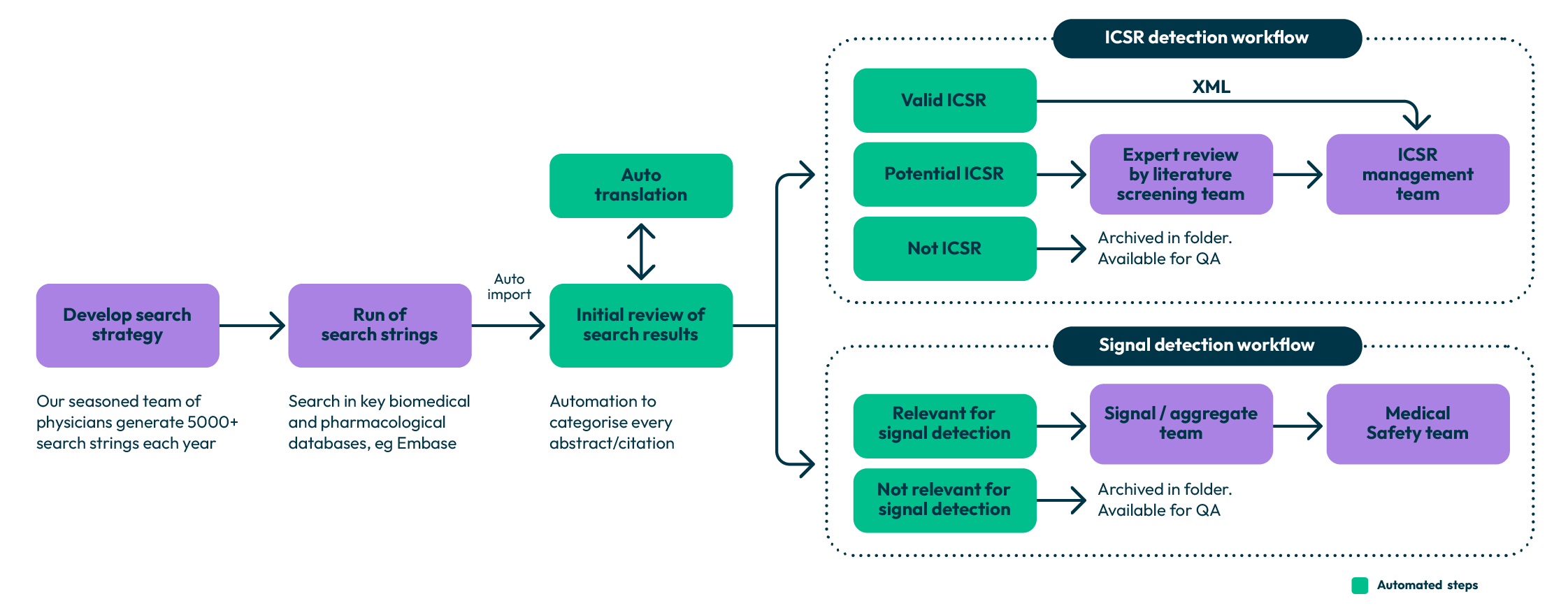
Our literature monitoring solutions at Qinecsa extend beyond standard databases. By combining the power of advanced technology with knowledgeable, hands-on experts, we create a robust, efficient system for identifying and processing safety information from scientific publications.
For example, this includes tracking peer-reviewed journals, conference abstracts, medical textbooks, regulatory bulletins, and specialised publications to ensure complete coverage of relevant safety information.
We also leverage artificial intelligence and natural language processing technologies to optimise literature search strategies. Our advanced algorithms continuously refine search parameters based on retrieved results. This dynamic approach improves detection sensitivity while reducing irrelevant results, thus creating a more efficient literature monitoring system.
Enhanced Efficiency and Integration
Technology alone cannot replace decision-making in literature evaluation. This is why our team of reviewers brings specialised therapeutic knowledge to the assessment of publications. Human expertise ensures that nuances and scientific implications are properly considered during the review process.
Qinecsa’s literature management solutions also integrate streamlined workflows that facilitate smooth transitions from literature identification to case processing. When safety information requiring reporting is identified, our system efficiently captures case data, ensuring regulatory timelines are met without unnecessary delays.
Value Added Case Management Solutions
Streamlined Review
Productivity Surge
Compliance and Efficiency
Efficient Signals
Streamlined workflow to enhance overall compliance and deliver inspection-ready solutions, saving time and resources for clients.
Working with Qinecsa for Your Needs
With Qinecsa managing your literature screening activities, you benefit from a scalable solution that adapts to your evolving products and global market presence. Our commitment to technological innovation and scientific excellence results in systems for our clients that not only meet current regulatory expectations, but anticipates future developments in pharmacovigilance.